
| PvPI |
|---|
Secretary-cum-Scientific Director

Dr. V. Kalaiselvan, Ph.D.
Secretary-cum-Scientific Director
Indian Pharmacopoeia Commission (IPC)
Ministry of Health & Family Welfare, Government of India
Brief Profile
Dr. V. Kalaiselvan is currently serving as the Secretary-cum-Scientific Director of the Indian Pharmacopoeia Commission (IPC), Ministry of Health & Family Welfare, Government of India. He holds a Ph.D. in Pharmaceutical Sciences from the University of Delhi. Dr. Kalaiselvan has been associated with IPC since 2009, contributing significantly to the domains of pharmacopoeial standards, rational use of medicines, drug safety, and regulatory systems.
He played a key role in the conceptualisation and nationwide implementation of the Pharmacovigilance Programme of India (PvPI) and the Materiovigilance Programme of India (MvPI), which have strengthened India’s surveillance framework for the safety of medicines and medical devices.
Dr. Kalaiselvan has represented IPC on various national and international platforms, including the World Health Organization (WHO), and continues to contribute to global initiatives for strengthening pharmacovigilance systems in low- and middle-income countries. In recognition of his work, the WHO Headquarters in Geneva acknowledged him as one of the significant contributors to global pharmacovigilance in their 50th Celebratory Album of the International Drug Monitoring Programme.
He has authored 11 chapters and published over 130 research and review articles in national and international journals. He is also a recipient of prestigious fellowships awarded by the Department of Science & Technology (DST) and the All India Council for Technical Education (AICTE).
With deep expertise in regulatory science and a forward-looking approach, Dr. Kalaiselvan brings strong leadership to IPC in its mission to ensure the quality, safety, and efficacy of medicines in India.
Dr Rajeev Singh Raghuvanshi
Secretary-cum-Scientific Director
INDIAN PHARMACOPOEIA COMMISSION
IPC, NCC-PvPI as WHO Collaborating Centre
Launch of IPC, NCC-PvPI as WHO Collaborating Centre for Pharmacovigilance on October 30, 2017
at
National Coordination Centre- Pharmacovigilance Programme of India, Indian Pharmacopoeia Commission, Ministry of Health and Family Welfare, Government of India
at
National Coordination Centre- Pharmacovigilance Programme of India, Indian Pharmacopoeia Commission, Ministry of Health and Family Welfare, Government of India
The National Coordination Centre- Pharmacovigilance Programme of India, Indian Pharmacopoeia Commission, Ministry of Health and Family Welfare, Government of India was launched as a WHO Collaborating Centre for Pharmacovigilance in Public Health Programmes and Regulatory Services on 30 October 2017.
The event also witnessed the launch of “National Strategic Plan for Scale up of Pharmacovigilance in India”; and “Pharmacovigilance Guidelines for Stakeholders”. As part of the event, a technical session on “WHO Global Patient Safety Challenge - Medication without Harm” was also organized.
Access to Medicines is a critical factor for achieving our public health goals and for success in the 2030 Sustainable Development Agenda. The issue of safe-guarding public health in India fully aligns with one of the three pillars of Universal Health Coverage; ensuring access to safe and quality medicines and vaccines in the country.
The Pharmacovigilance Programme of India (PvPI) has progressed considerably in the last few years. The Pharmacovigilance Programme of India (PvPI) was approved by the Ministry of Health and Family Welfare (MOHFW), Government of India (GOI) in July 2010 with the primary objective of the programme to create a nation-wide system for patient safety reporting. There are 250 functioning Adverse Drug Monitoring centres in the country (in medical colleges and corporate hospitals) as part of the Pharmacovigilance Programme of India.
In his inaugural address, Dr. R. K. Vats, Additional Secretary, MoHFW said that “Integrating pharmacovigilance as an essential component of public health programmes (PHPs) is crucial for patient safety.” Reflecting the government’s strong commitment, he added “The Honourable Prime Minister of India, Shri Narendra Modi has a vision focusing on the universal health care agenda so as to provide all Indian citizens, regardless of their economic, social or cultural backgrounds the right to affordable, and quality medical products.” On behalf of MoHFW, he assured to extend all kind of support to PvPI.
The pharmaceutical sector is one of the key 25 sectors identified by the Government of India under the ambitious ‘Make in India’ initiative, which is likely to provide the necessary impetus to the sector in order to achieve its true potential. At present, the Indian pharmaceuticals industry is third largest in volume and the tenth largest in value, globally.
Dr Clive Ondari, Coordinator, Safety & Vigilance, WHO “India’s concerted efforts in the area of pharmacovigilance have been recognized by WHO. It has also been highlighted that the country needs to play a bigger role in the global landscape of adverse drug reaction (ADR) monitoring, thus making India a hub for pharmacovigilance in Public Health Programmes and Regulatory Services. This would be the first WHO CC on this theme globally and the first in the entire WHO South East Asia Region”.
The National Regulatory Authority of India (NRA) competencies were reiterated with the WHO Global Benchmarking Tool during a comprehensive review by WHO led team of international experts from 13-17 February 2017. The Indian NRA was declared functional with Pharmacovigilance as one of the core functions with a maturity level of 4 in the WHO global NRA benchmarking undertaken in February 2017.
Dr G.N. Singh, Secretary-Cum-Scientific Director, IPC; and Drugs Controller General (India) during inaugural said, “Pharmacovigilance and drug safety monitoring is of pivotal importance, for improving treatment support and adherence. The Pharmacovigilance Programme of India and its integration with public health programmes has been noteworthy. It is vital to ensure that adequate systems and practices for reporting of the Adverse Drug Reactions are in place to ensure that the benefit of use of medicine outweighs the risks associated with its use.”
Indian Pharmacopoeia Commission through National Coordination Centre, Pharmacovigilance Programme of India is one of the active member countries in WHO-Programme of International Drug Monitoring and is also leading the thematic area of Vigilance as part of the South East Asia Regulatory Network (SEARN).
Speaking at the inaugural ceremony of the meeting, Ms Prakin Suchaxaya, Coordinator, Health Programmes, WHO Country Office for India said “WHO has been playing a pivotal role in supporting countries in strengthening the pharmacovigilance systems for medical products and in promoting equitable access to quality, safe, efficacious, and affordable medical products. WHO also provides technical and operational assistance towards strengthening of India’s National Regulatory Authority (NRA). The endeavour is to scale-up the support to public health programmes and develop a strong regulatory network for India and South East Asian Countries”.
Other prominent dignitaries at the inaugural were: Padamshree Dr Nitya Anand, Dr KK Aggarwal, Dr Shanthi Pal, WHO headquarters, Dr Raj Long, Bill and Melinda Gates Foundation, Dr Manisha Shridhar, WHO SEARO, Dr Hilde De Greave, and Dr Madhur Gupta, WHO India Country Office.
An international team of experts from WHO headquarters, South East Asia Regional Office, WHO Country Office, senior officials from the MoHFW, officials from CDSCO and its affiliated institutions, various public health programme stakeholders such as Adverse Event Following Immunization (AEFI), Revised National Tuberculosis Control Programme (RNTCP), National AIDS Control Organization (NACO), National Vector-Borne Disease Control Programme (NVBDCP), Indian Medical Association (IMA), Indian Council of Medical Research (ICMR), National Accreditation Board for Hospitals (NABH), Adverse Drug Reaction Monitoring Centre (AMC) Coordinators, Industry representatives, relevant stakeholders, and Pharmacovigilance Programme of India officials were present at the occasion.
Introduction & Function
Introduction
The Central Drugs Standard Control Organisation (CDSCO), New Delhi, under the aegis of Ministry of Health & Family Welfare, Government of India has initiated a nation-wide pharmacovigilance programme in July, 2010, with the All India Institute of Medical Sciences (AIIMS), New Delhi as the National Coordinating Centre (NCC) for monitoring Adverse Drug Reactions (ADR) in the country to safe-guard Public Health. In year 2010, 22 ADR monitoring centres (AMCs) including AIIMS, New Delhi had been set up under this Programme. To ensure implementation of this programme in a more effective way, the National Coordinating Centre was then shifted from the All India Institute of Medical Sciences (AIIMS), New Delhi to the Indian Pharmacopoeia Commission (IPC), Ghaziabad, (U.P.) in April, 2011.
The mission of PvPI is to safeguard the health of the Indian population by ensuring that the benefit of use of medicine outweighs the risks associated with its use. Since there exist considerable social and economic consequences of adverse drug reactions and the positive benefit/cost ratio of implementing appropriate risk management - there is a need to engage healthcare professionals and the public at large, in a well structured programme to build synergies for monitoring adverse drug reactions in the country.
The purpose of the PvPI is to collate data, analyze it and use the inferences to recommend informed regulatory interventions, besides communicating risks to healthcare professionals and the public. The broadened patient safety scope of pharmacovigilance includes the detection of medicines of substandard quality as well as prescribing, dispensing and administration errors. Counterfeiting, antimicrobial resistance, and the need for real time surveillance in mass vaccinations are other pharmacovigilance challenges which need to be addressed.
The vision of PvPI is to improve patient safety and welfare in Indian population by monitoring drug safety and thereby reducing the risk associated with use of medicines.The ultimate safety decisions on medicines may need considerations of comparative benefit/risk evaluations between products for similar indications, so the complexity is great.
Scope and Objectives
- To create a nation-wide system for patient safety reporting
- To identify and analyse new signal from the reported cases
- To analyse the benefit - risk ratio of marketed medications
- To generate evidence based information on safety of medicines
- To support regulatory agencies in the decision-making process on use of medications
- To communicate the safety information on use of medicines to various stakeholders to minimise the risk
- To emerge as a national centre of excellence for pharmacovigilance activities
- To collaborate with other national centres for the exchange of information and data management
- To provide training and consultancy support to other national pharmacovigilance centres across globe
- To promote rational use of medicine
Short Term Goals
- To develop and implement pharmaco-vigilance system in India
- To enrol, initially, all MCI approved medical colleges in the program covering north, south, east and west of India
- To encourage healthcare professionals in reporting of adverse reaction to drugs, vaccines, medical devices and biological products
- Collection of case reports and data
Long Term Goals
- To expand the pharmacovigilance programme to all hospitals (govt. & private) and centres of public health programs located across India
- To develop and implement electronic reporting system (e-reporting)
- To develop reporting culture amongst healthcare professionals
- To make ADR reporting mandatory for healthcare professionals
Organogram of NCC
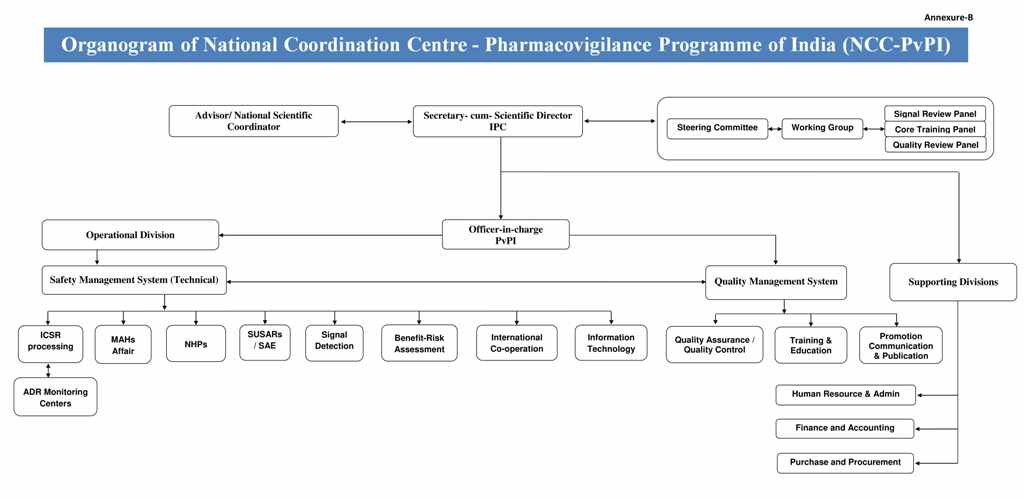
The Functions of a National Pharmacovigilance System have been defined to include the following:
- To create a nation-wide system for patient safety reporting
- To identify and analyse the new signal (ADR) from the reported cases
- To analyse the benefit - risk ratio of marketed medications
- To generate the evidence based information on safety of medicines
- To support regulatory agencies in the decision-making process on use of medications
- To communicate the safety information on use of medicines to various stakeholders to minimise the risk
- To emerge as a national centre of excellence for Pharmacovigilance activities
- To collaborate with other national centres for the exchange of information and data management
- To provide training and consultancy support to other National Pharmacovigilance Centres located across globe
Minimum requirements for a functional Pharmacovigilance system
Pharmacovigilance activities may be undertaken by several organizations, individuals and agencies. The Pharmacovigilance Programme of India fulfills the minimum requirements that should be present in any functional national pharmacovigilance system, as per WHO, which include the following:
- A national pharmacovigilance centre with designated staff (at least one full time), stable basic funding, clear mandates, well defined structures and roles and collaborating with the WHO Programme for International Drug Monitoring (The National Coordinating Centre for PvPI being The Indian Pharmacopoeia Commission, Ghaziabad, under Ministry of Health & Family Welfare, Government of India)
- The existence of a national spontaneous reporting system with a national individual case safety report (ICSR) form i.e. an adverse drug reaction (ADR) reporting form
- A national database or system for collating and managing ADR reports (Vigibase database and Vigiflow software for PvPI)
- A national ADR or pharmacovigilance advisory committee able to provide technical assistance on causality assessment, risk assessment, risk management, case investigation and where necessary, crisis management including crisis communication (Steering Committee provides technical assistance in PvPI)
- A clear strategy for routine and crisis communications
Network
Data Flow
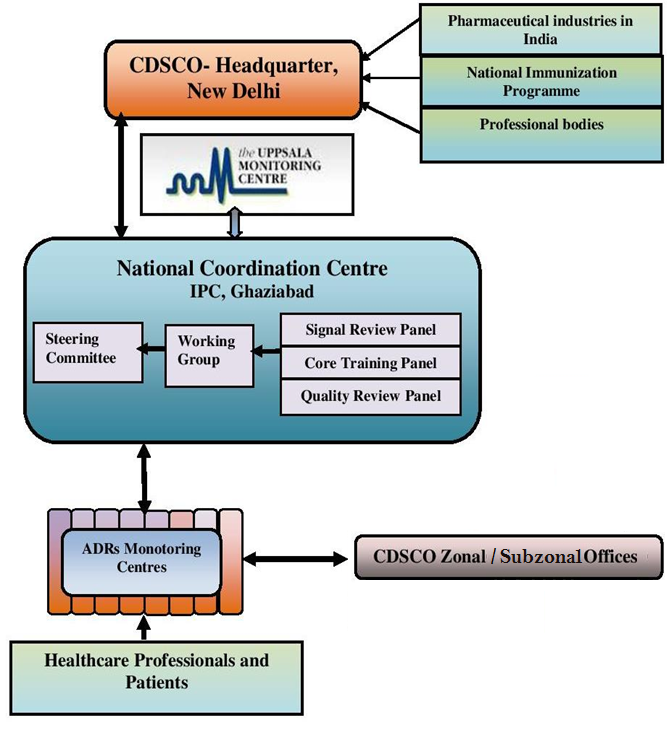
Once the Medical institute is enrolled as an AMC under PvPI, the AMC starts reporting ICSRs to NCC via a VigiFlow. These ICSRs are then assessed at NCC for quality of data and if found valid, they are further committed to the global drug monitoring centre “Uppsala Monitoring Centre” in Sweden. But if the data is not complete or valid, then the ICSRs are reverted back to their concerned AMC with the query or necessary comments, so that the respective ICSR can be corrected or completed and sent to NCC again for evaluation. The data from NCC is also sent to CDSCO, as and when required.
Following chart explains the flow of data at regional, national and international level:
Assessment of Individual Case Safety Reports
The quality of the ICSR is assessed for completeness of information and is reviewed for:
- Quality of documentation: e.g. completeness and integrity of data, quality of diagnosis, follow-up.
- Coding: Drug names should be registered in a systematic way, for example by using the WHO Drug Dictionary (which is based on the ATC classification). For the coding of the adverse events the WHO Adverse Reaction Terminology (WHOART) and internationally recognised terminology (e.g. MedDRA) should be used.
- Relevance with regard to the detection of new reactions, drug regulation, or scientific or educational value. The following questions especially may be asked:
- New drug - a new drug shall continue to be considered as new drug for a period of four years from the date of its first approval or its inclusion in IP, whichever is earlier.
- Unknown reaction- Not included in the approved Summary of Product Characteristics
- Serious reaction- Results into either death, life-threatening condition, hospitalisation or prolonged hospitalisation, disability, congenital anomaly, required intervention to prevent permanent impairment/damage or any other medically important condition
- Identification of duplicate reports: Certain characteristics of a case (sex, age or date of birth, dates of drug exposure, etc.) may be used to identify duplicate reporting.
- Causality assessment:With few exceptions, case reports describe suspected adverse drug reactions. The likelihood of a causal relationship between drug exposure and adverse events must be validated.
Utilization of the Data
Data collected in Pharmacovigilance can be used in a variety of ways:
- Signal generation and strengthening: A major aim of pharmacovigilance is the early detection of signals with regard to possible adverse reactions. A signal may be strengthened by further analysis can help the regulatory system in performing regulatory activities.
- Drug regulation: After approval of a medicinal product, all available domestic and international safety information is continuously monitored by the drug regulatory authority and MAH. The PvPI data can be useful in resolving the problems by adaptation of the approved product information (inclusion of new adverse effects and warnings).
- Education: The information from PvPI data is useful in updating the knowledge of healthcare professionals during continuous medical education programme on pharmacovigilance. Signal Review Panel under PvPI
Contacts
Ministry of Health & Family Welfare
Govt. of India
Sector-23, Raj Nagar, Ghaziabad-201 002.
Tel.:0120-2783400, 2783401, 2783392,
FAX: 0120-2783311
Email: lab.ipc@gov.in, pvpi.ipcindia@gmail.com,
pvpi@ipcindia.net

Copyright Policy | Privacy Policy | Hyperlink Policy | Disclaimer | Terms and Condition | Organisations Involved | Help
Copyright @ 2017 IPC All Rights Reserved
You are Visitor No.
Last updated: 31/10/2018 12:30:02